Hydrogen inhalation therapy is effective in saving lives and improving prognosis in patients with out-of-hospital cardiac arrest
At our hospital, we perform aging treatment as the following three arrows:
① NMN (nicotinamide mononucleotide) therapy
② Human-derived mesenchymal stem cell culture supernatant (exosome) therapy
③ Hydrogen gas inhalation therapy.
We use them to treat age-related changes in the body from the cellular and even mitochondrial level.
The other day, a patient asked me, “Which is better, inhaling hydrogen gas or drinking hydrogen water?”
Based on my own experience, I answered, “There is an effect of hydrogen gas inhalation.”
There are people who drink hydrogen water regularly, so we do not deny its effects.
However, “hydrogen gas inhalation therapy” is certainly effective.

Hydrogen can selectively eliminate hydroxyl radicals, which are the most oxidizing reactive oxygen species.
The mechanism by which hydrogen suppresses inflammation was not well understood.
Several publications have shown that the effects of hydrogen in animal models of inflammation are based on the inhibition of mitochondrial oxidation.
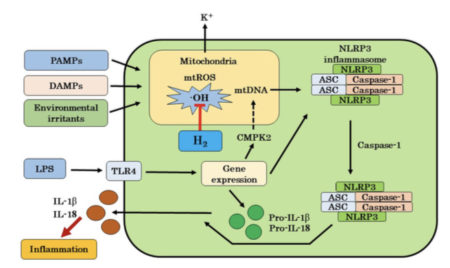
Hydrogen is the only substance that can enter mitochondria and eliminate hydroxyl radicals, so only hydrogen can cure chronic inflammatory diseases and many diseases related to chronic inflammation.

Hydrogen is an inert substance.
However, inhalation of 2% hydrogen gas after resuscitation of cardiac arrest patients can be expected to restore brain function in the actual clinical setting.
Hydrogen is used to remove reactive oxygen species generated during ischemia-reperfusion in the brain, thereby minimizing damage to brain tissue and leading to recovery of brain function.



Efficacy of inhaled hydrogen on neurological outcome following brain ischaemia during post-cardiac arrest care (HYBRID II): a multi-centre, randomised, double-blind, placebo-controlled trial
eClinicalMedicine 2023;58: 101907
Background
Inhaled molecular hydrogen gas (H2) has been shown to improve outcomes in animal models of cardiac arrest (CA). H2 inhalation is safe and feasible in patients after CA. We investigated whether inhaled H2 would improve outcomes after out-of-hospital CA (OHCA).
Methods
HYBRID II is a prospective, multicentre, randomised, double-blind, placebo-controlled trial performed at 15 hospitals in Japan, between February 1, 2017, and September 30, 2021. Patients aged 20–80 years with coma following cardiogenic OHCA were randomly assigned (1:1) using blinded gas cylinders to receive supplementary oxygen with 2% H2 or oxygen (control) for 18 h. The primary outcome was the proportion of patients with a 90-day Cerebral Performance Category (CPC) of 1 or 2 assessed in a full-analysis set. Secondary outcomes included the 90-day score on a modified Rankin scale (mRS) and survival. HYBRID II was registered with the University Hospital Medical Information Network (registration number: UMIN000019820) and re-registered with the Japan Registry for Clinical Trials (registration number: jRCTs031180352).
Findings
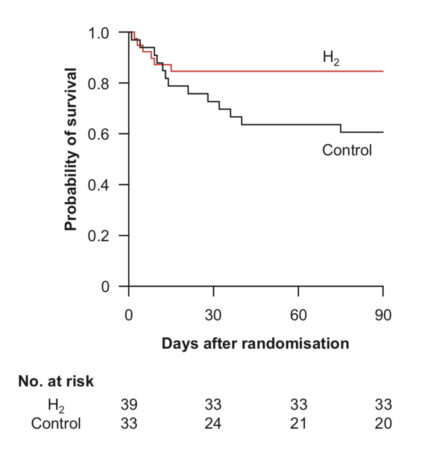
The trial was terminated prematurely because of the restrictions imposed on enrolment during the COVID-19 pandemic. Between February 1, 2017, and September 30, 2021, 429 patients were screened for eligibility, of whom 73 were randomly assigned to H2 (n = 39) or control (n = 34) groups. The primary outcome, i.e., a CPC of 1 or 2 at 90 days, was achieved in 22 (56%) and 13 (39%) patients in the H2 and control groups (relative risk compared with the control group, 0.72; 95% CI, 0.46–1.13; P = 0.15), respectively. Regarding the secondary outcomes, median mRS was 1 (IQR: 0–5) and 5 (1–6) in the H2 and control groups, respectively (P = 0.01). An mRS score of 0 was achieved in 18 (46%) and 7 (21%) patients in the H2 and control groups, respectively (P = 0.03). The 90-day survival rate was 85% (33/39) and 61% (20/33) in the H2 and control groups, respectively (P = 0.02).
Interpretation
The increase in participants with good neurological outcomes following post-OHCA H2 inhalation in a selected population of patients was not statistically significant. However, the secondary outcomes suggest that H2 inhalation may increase 90-day survival without neurological deficits.